 Senin, 10 Februari 2025 (06:44)
Senin, 10 Februari 2025 (06:44)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
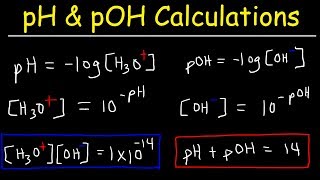
Chapter 10 Exercises 7 and 8 Determine Acidity or Basicity from H3O+ or OH- Concentrations (Timothy Siniscalchi) View |
 |
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems (The Organic Chemistry Tutor) View |
 |
How to find pH, pOH, H3O+, and OH- STEP BY STEP (Melissa Maribel) View |
![Download Lagu What is the [OH-] for a solution with a pH of 8.0 Thumbnail](https://img.youtube.com/vi/4XKXayPo-BQ/mqdefault.jpg) |
What is the [OH-] for a solution with a pH of 8.0 (Jeff Lehman) View |
![Download Lagu ACIDS,BASES AND SALTS:At 25ºC,[H+]= 4.6 x 10-8 M.Find [OH–].Is the solution acidic/basic or neutral Thumbnail](https://img.youtube.com/vi/2fiRuTqaXYE/mqdefault.jpg) |
ACIDS,BASES AND SALTS:At 25ºC,[H+]= 4.6 x 10-8 M.Find [OH–].Is the solution acidic/basic or neutral (ZULUBA CONSULTANCY) View |
![Download Lagu pH, pOH, [H3O+] and [OH-] Related Calculations (Chemistry) Thumbnail](https://img.youtube.com/vi/BL35bCSB4P0/mqdefault.jpg) |
pH, pOH, [H3O+] and [OH-] Related Calculations (Chemistry) (Joy Oyebisi Tutoring) View |
 |
pH and pOH in Acids, Bases and Water 07022020 (Chemistry Lecture) View |
 |
pKa, Ka, and Acid Strength (The Organic Chemistry Tutor) View |
 |
Acids and Bases - Relationship between pH, pOH and Kw (Stoddard Tutoring) View |
![Download Lagu Chemistry 30 - Find [h3o+] using Ka Thumbnail](https://img.youtube.com/vi/q9QofYlffP8/mqdefault.jpg) |
Chemistry 30 - Find [h3o+] using Ka (Mayay) View |